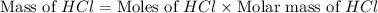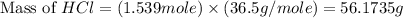Chemistry, 27.06.2019 19:30 helpmeplz11239
Determine the theoretical yield of hcl if 60.0 g of bc13 and 37.5 g of h20 are reacted according to the following balanced reaction. a possibly useful molar mass is bc13 117.16 g/mol. bc13(g)+3 h20(1) -- h3bo3(s)+3 hc1(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Determine the theoretical yield of hcl if 60.0 g of bc13 and 37.5 g of h20 are reacted according to...
Questions

Social Studies, 21.07.2019 14:30





Mathematics, 21.07.2019 14:30





History, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30


Mathematics, 21.07.2019 14:30




Social Studies, 21.07.2019 14:30


History, 21.07.2019 14:30

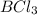 = 60 g
= 60 g
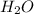 = 37.5 g
= 37.5 g
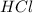 = 36.5 g/mole
= 36.5 g/mole
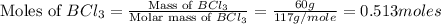
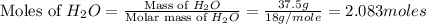
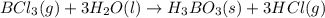
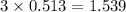 moles of
moles of