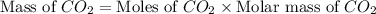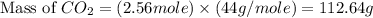Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1
You know the right answer?
Give the theoretical yield, in grams, of co2 from the reaction of 4.000 moles of c8h18 with 4.000 mo...
Questions


Mathematics, 05.05.2020 13:03

Mathematics, 05.05.2020 13:03



Biology, 05.05.2020 13:03

English, 05.05.2020 13:03

English, 05.05.2020 13:03




Mathematics, 05.05.2020 13:03

Mathematics, 05.05.2020 13:03



Social Studies, 05.05.2020 13:03


Business, 05.05.2020 13:03


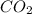 is, 112.64 grams.
is, 112.64 grams. = 4 moles
= 4 moles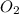 = 4 moles
= 4 moles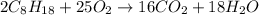
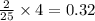 moles of
moles of 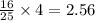 moles of
moles of