
Given that the density of the saturated solution is found to be 1.16 g/ml. the molar mass of copper sulfate pentahydrate is 249.68 g/mol, calculate grams of copper sulfate pentahydrate that will dissolve in 100 g of water at 0oc (show calculations for full credit)

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3
You know the right answer?
Given that the density of the saturated solution is found to be 1.16 g/ml. the molar mass of copper...
Questions

Mathematics, 24.03.2021 02:20

Mathematics, 24.03.2021 02:20



Mathematics, 24.03.2021 02:20


Mathematics, 24.03.2021 02:20


Social Studies, 24.03.2021 02:20

Mathematics, 24.03.2021 02:20

Mathematics, 24.03.2021 02:20

Biology, 24.03.2021 02:30


English, 24.03.2021 02:30

English, 24.03.2021 02:30


Social Studies, 24.03.2021 02:30



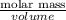
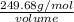
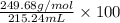
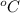 .
. 

