
Chemistry, 29.01.2020 19:02 Pizzapegasus1
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to water vapor at 110.0 °c. the specific heats of ice, water, and steam are 2.09 j/g-k, 4.18 j/g-k, and 1.84 j/g-k, respectively. for , δhfus = 6.01 kj/mol and δhvap = 40.67 kj/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1
You know the right answer?
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to wat...
Questions

Chemistry, 25.01.2021 14:00

History, 25.01.2021 14:00


English, 25.01.2021 14:00


Physics, 25.01.2021 14:00

History, 25.01.2021 14:00

Mathematics, 25.01.2021 14:00


History, 25.01.2021 14:00

English, 25.01.2021 14:00

Mathematics, 25.01.2021 14:00



Mathematics, 25.01.2021 14:00

English, 25.01.2021 14:00



Mathematics, 25.01.2021 14:00

English, 25.01.2021 14:00

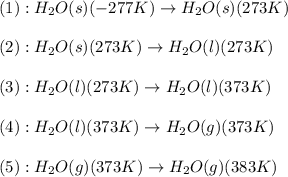
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0482/9975/e4ef0.png)
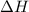 = enthalpy change = ?
= enthalpy change = ?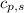 = specific heat of solid water = 2.09 J/gk
= specific heat of solid water = 2.09 J/gk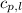 = specific heat of liquid water = 4.18 J/gk
= specific heat of liquid water = 4.18 J/gk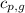 = specific heat of liquid water = 1.84 J/gk
= specific heat of liquid water = 1.84 J/gk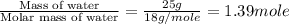
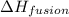 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole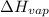 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[25g\times 4.18J/gK\times (273-277)k]+1.39mole\times 6010J/mole+[25g\times 2.09J/gK\times (373-273)k]+1.39mole\times 40670J/mole+[25g\times 1.84J/gK\times (383-373)k]](/tpl/images/0482/9975/b0606.png)
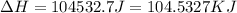 (1 KJ = 1000 J)
(1 KJ = 1000 J)

