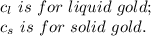Chemistry, 28.06.2019 18:30 ilizzy1224
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg of liquid gold at an initial temperature at 1336k. the solid and liquid other. when the two reach thermal equilibrium will the mixture be entirely solid, or will they be in a mixed solid/liquid phase? explain how you know. draw two separate temp. vs. energy added diagrams to you answer this question. can only exchange heat with each

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg...
Questions

Engineering, 27.10.2020 22:40




Mathematics, 27.10.2020 22:40


Mathematics, 27.10.2020 22:40



SAT, 27.10.2020 22:40




Mathematics, 27.10.2020 22:40

English, 27.10.2020 22:40




Mathematics, 27.10.2020 22:40

Mathematics, 27.10.2020 22:40