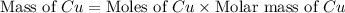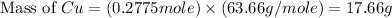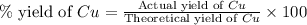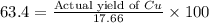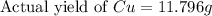Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3
You know the right answer?
If 5.00 grams of aluminum react with an excess of copper (ii) sulfate and the percentage yield is 63...
Questions


Mathematics, 23.06.2021 06:40


Mathematics, 23.06.2021 06:40

Mathematics, 23.06.2021 06:40

English, 23.06.2021 06:40

Mathematics, 23.06.2021 06:40

Mathematics, 23.06.2021 06:50



Physics, 23.06.2021 06:50

History, 23.06.2021 06:50

Social Studies, 23.06.2021 06:50

Mathematics, 23.06.2021 06:50

English, 23.06.2021 06:50



World Languages, 23.06.2021 06:50


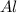 = 5 g
= 5 g
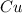 = 63.66 g/mole
= 63.66 g/mole
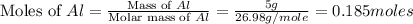
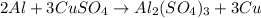
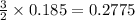 moles of
moles of