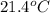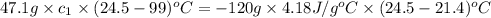Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1


Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
A47.1 g sample of a metal is heated to 99.0°c and then placed in a calorimeter containing 120.0 g of...
Questions

Biology, 13.11.2020 18:50


Physics, 13.11.2020 18:50

Geography, 13.11.2020 18:50

Mathematics, 13.11.2020 18:50


English, 13.11.2020 18:50


History, 13.11.2020 18:50


Mathematics, 13.11.2020 18:50

Health, 13.11.2020 18:50

Mathematics, 13.11.2020 18:50

History, 13.11.2020 18:50


Mathematics, 13.11.2020 18:50

Computers and Technology, 13.11.2020 18:50

Mathematics, 13.11.2020 18:50

History, 13.11.2020 18:50

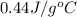 ).
).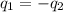
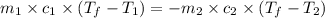
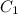 = specific heat of metal = ?
= specific heat of metal = ?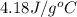
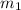 = mass of metal = 47.1 g
= mass of metal = 47.1 g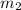 = mass of water = 120 g
= mass of water = 120 g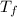 = final temperature of water =
= final temperature of water = 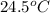
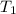 = initial temperature of metal =
= initial temperature of metal = 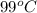
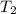 = initial temperature of water =
= initial temperature of water =