
Chemistry, 28.06.2019 20:20 joseroblesrivera123
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is the net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is zn (s) + 2h+ (aq) → zn2+ (aq) + h2 (g) 2zn (s) + h+ (aq) → 2zn2+ (aq) + h2 (g) zn (s) + 2hbr (aq) → znbr2 (aq) + 2h+ (aq) zn (s) + 2br- (aq) → znbr2 (aq) zn (s) + 2hbr (aq) → znbr2 (s) + 2h+ (aq)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is the net ion...
Questions










Arts, 17.03.2021 23:40


Computers and Technology, 17.03.2021 23:40





Mathematics, 17.03.2021 23:40

Biology, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

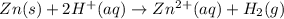
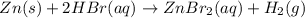
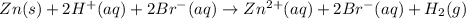
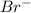 ion is the spectator ions.
ion is the spectator ions.


