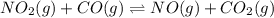Chemistry, 28.06.2019 21:20 tamikalove78
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume of the system is decreased at constant temperature, what change will this cause in the system? a shift to produce more no a shift to produce more co a shift to produce more no2 no shift will occur

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume...
Questions



History, 20.09.2020 06:01



Mathematics, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01


Mathematics, 20.09.2020 06:01


History, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01

Physics, 20.09.2020 06:01


History, 20.09.2020 06:01


Mathematics, 20.09.2020 06:01

Chemistry, 20.09.2020 06:01