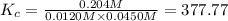Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equilibrium concentrations for the reaction between carbon monoxide and molecular chlorine to form carbonyl chloride at a certain temperature are [co] = 0.0210 m, [cl2] = 0.0450 m, and [cocl2] = 0.204 m. co(g) + cl2(g) ⇆ cocl2(g) calculate the equilibrium constant (kc).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equ...
Questions

History, 01.06.2020 11:57

Biology, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57

Chemistry, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57

Geography, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57

English, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57

Biology, 01.06.2020 11:57

Mathematics, 01.06.2020 11:57






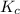
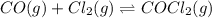
![[CO] = 0.0210 M](/tpl/images/0028/7944/23f4d.png)
![[Cl_2] = 0.0450 M](/tpl/images/0028/7944/558aa.png)
![[COCl_2] = 0.204 M](/tpl/images/0028/7944/1309b.png)
![K_c=\frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0028/7944/36d91.png)