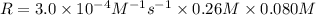Chemistry, 28.06.2019 23:30 springcoates
3. the rate law for the reaction nh4+(aq) + no2–(aq) → n2(g) + 2h2o(l) is given by rate = k[nh4+][no2–]. at 25ºc, the rate constant is 3.0 × 10–4/ m · s. calculate the rate of the reaction at this temperature if [nh4+] = 0.26 m and [no2–] = 0.080 m. (5 points)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
3. the rate law for the reaction nh4+(aq) + no2–(aq) → n2(g) + 2h2o(l) is given by rate = k[nh4+][no...
Questions


Social Studies, 08.02.2021 17:10

Mathematics, 08.02.2021 17:10

Spanish, 08.02.2021 17:10


Mathematics, 08.02.2021 17:10



English, 08.02.2021 17:10


History, 08.02.2021 17:10


Mathematics, 08.02.2021 17:10

History, 08.02.2021 17:10



French, 08.02.2021 17:10

Mathematics, 08.02.2021 17:10


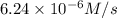 .
.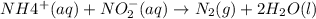
![[NH_4^{+}]=0.26 M](/tpl/images/0028/9019/f2f2d.png)
![[NO_2^{-}]=0.080 M](/tpl/images/0028/9019/214e5.png)
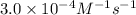
![R= k[NH_{4}^+][NO_{2}^-]](/tpl/images/0028/9019/cb361.png)