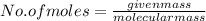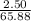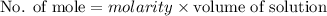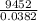Chemistry, 29.06.2019 00:20 webbjalia04
A2.50 g sample of powdered zinc is added to 100.0 ml of a 2.00 m aqueous solution of hydrobromic acid in a calorimeter. the total heat capacity of the calorimeter and solution is 448 j/k. the observed increase in temperature is 21.1 k at a constant pressure of one bar. calculate the standard enthalpy of reaction using these data. zn(s)+2hbr(aq)⟶znbr2(aq)+h2(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1
You know the right answer?
A2.50 g sample of powdered zinc is added to 100.0 ml of a 2.00 m aqueous solution of hydrobromic aci...
Questions




Health, 01.09.2019 18:10

Mathematics, 01.09.2019 18:10

History, 01.09.2019 18:10

Spanish, 01.09.2019 18:10

Social Studies, 01.09.2019 18:10

Biology, 01.09.2019 18:10




Business, 01.09.2019 18:10

Chemistry, 01.09.2019 18:10

Geography, 01.09.2019 18:10





History, 01.09.2019 18:10