
Calculate the number of pounds of co2co2 released into the atmosphere when a 22.0 gallon22.0 gallon tank of gasoline is burned in an automobile engine. assume that gasoline is primarily octane, c8h18,c8h18, and that the density of gasoline is 0.692 g⋅ml−1.0.692 g⋅ml−1. this assumption ignores additives. also, assume complete combustion. co2co2 released:

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
Calculate the number of pounds of co2co2 released into the atmosphere when a 22.0 gallon22.0 gallon...
Questions


Business, 16.10.2021 03:10


Mathematics, 16.10.2021 03:10

Social Studies, 16.10.2021 03:10


Mathematics, 16.10.2021 03:10

Mathematics, 16.10.2021 03:10

Mathematics, 16.10.2021 03:10


SAT, 16.10.2021 03:10




Spanish, 16.10.2021 03:10


Mathematics, 16.10.2021 03:10



Biology, 16.10.2021 03:10

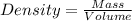
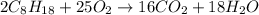
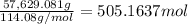
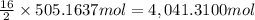 of carbon-dioxide
of carbon-dioxide

