
Chemistry, 29.06.2019 03:10 fifthward92
An open flask sitting in a lab fridge looks empty, but it is actually filled with a mixture of gases called air. if the flask volume is 2.50 l, and the air is at standard temperature and pressure, how many gaseous molecules does the flask contain?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1


Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
An open flask sitting in a lab fridge looks empty, but it is actually filled with a mixture of gases...
Questions

Chemistry, 02.04.2021 05:40

Geography, 02.04.2021 05:40

Mathematics, 02.04.2021 05:40



Computers and Technology, 02.04.2021 05:40


Chemistry, 02.04.2021 05:40



Health, 02.04.2021 05:40


Mathematics, 02.04.2021 05:40

Mathematics, 02.04.2021 05:40

Mathematics, 02.04.2021 05:40


English, 02.04.2021 05:40

Mathematics, 02.04.2021 05:40


Biology, 02.04.2021 05:40

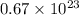 molecules are contained in the flask.
molecules are contained in the flask.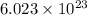 of particles.
of particles.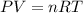
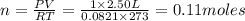
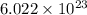 molecules
molecules 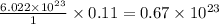 molecules
molecules

