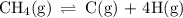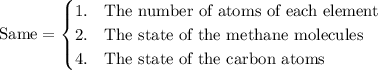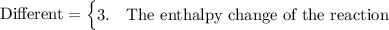Ch4 (g) yields c(g) + 4h (g) (reaction for expansion)
compare the reaction for the "expansion...

Ch4 (g) yields c(g) + 4h (g) (reaction for expansion)
compare the reaction for the "expansion" of methane with the reverse of the reaction that represents the standard enthalpy of formation. which properties are the same for both reactions and which are different?
1. the number of atoms of each element, 2. the state of the methane molecules, 3. the enthalpy change of the reaction, 4. the state of the carbon atoms.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
Questions

Health, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00



World Languages, 19.03.2021 14:00


History, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00

Computers and Technology, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00

Biology, 19.03.2021 14:00

History, 19.03.2021 14:00