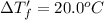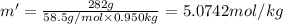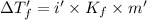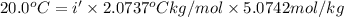Chemistry, 01.07.2019 16:10 whitneyb13
This problem has been solved! see the answerwhen 282. g of glycine (c2h5no2) are dissolved in 950. g of a certain mystery liquid x, the freezing point of the solution is 8.2c lower than the freezing point of pure x. on the other hand, when 282. g of sodium chloride are dissolved in the same mass of x, the freezing point of the solution is 20.0c lower than the freezing point of pure x. calculate the van't hoff factor for sodium chloride in x.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
This problem has been solved! see the answerwhen 282. g of glycine (c2h5no2) are dissolved in 950. g...
Questions

Mathematics, 07.10.2019 21:30

Health, 07.10.2019 21:30


Social Studies, 07.10.2019 21:30

English, 07.10.2019 21:30





History, 07.10.2019 21:30


History, 07.10.2019 21:30

Mathematics, 07.10.2019 21:30

Mathematics, 07.10.2019 21:30

English, 07.10.2019 21:30




History, 07.10.2019 21:30

Social Studies, 07.10.2019 21:30

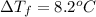
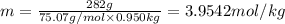
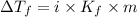
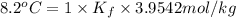
 ..(1)
..(1)