Chemistry, 01.07.2019 16:10 richardgibson2005
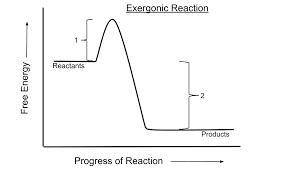
Which of the following is true for all exergonic reactions? the reaction releases energy. a net input of energy from the surroundings is required for the reactions to proceed. the reactions are rapid. the products have more total energy than the reactants. the reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3
You know the right answer?
Which of the following is true for all exergonic reactions? the reaction releases energy. a net inp...
Questions

Mathematics, 02.04.2020 01:25

Mathematics, 02.04.2020 01:26

Chemistry, 02.04.2020 01:26

Advanced Placement (AP), 02.04.2020 01:26

Mathematics, 02.04.2020 01:26


Mathematics, 02.04.2020 01:26

Mathematics, 02.04.2020 01:26

English, 02.04.2020 01:26






Computers and Technology, 02.04.2020 01:26

Mathematics, 02.04.2020 01:26

Biology, 02.04.2020 01:26

Mathematics, 02.04.2020 01:26


Mathematics, 02.04.2020 01:26