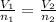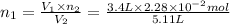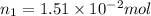Nitrogen dioxide is a red-brown gas responsible for the brown color of smog. a container of nitrogen dioxide that is at low pressure and at room temperature has a volume of 3.41l. after more nitrogen dioxide is added, the container holds 2.28×10−2mol of nitrogen dioxide and the volume of the container is 5.11l, still at the same pressure and temperature. how many moles of nitrogen dioxide were in the container initially? give your answer in three significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1

Chemistry, 23.06.2019 10:00
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1

You know the right answer?
Nitrogen dioxide is a red-brown gas responsible for the brown color of smog. a container of nitrogen...
Questions



Computers and Technology, 02.03.2021 20:40

Mathematics, 02.03.2021 20:40




Computers and Technology, 02.03.2021 20:40


Biology, 02.03.2021 20:40


Mathematics, 02.03.2021 20:40

Mathematics, 02.03.2021 20:40

Mathematics, 02.03.2021 20:40


Mathematics, 02.03.2021 20:40


Social Studies, 02.03.2021 20:40

Mathematics, 02.03.2021 20:40

Chemistry, 02.03.2021 20:40

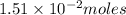 of nitrogen dioxide were in the container .
of nitrogen dioxide were in the container .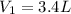
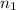
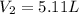
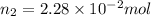
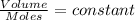 (at constant pressure and temperature)
(at constant pressure and temperature)