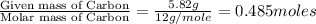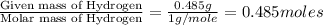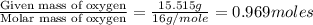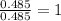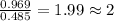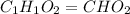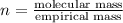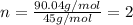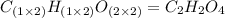Chemistry, 01.07.2019 21:20 robert7248
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 21.33 grams of co2 and 4.366 grams of h2o are produced. in a separate experiment, the molar mass is found to be 90.04 g/mol. determine the empirical formula and the molecular formula of the organic compound.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions


Mathematics, 12.12.2020 17:00

English, 12.12.2020 17:00

Chemistry, 12.12.2020 17:00

Social Studies, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

English, 12.12.2020 17:00

English, 12.12.2020 17:00




Business, 12.12.2020 17:00



English, 12.12.2020 17:00

History, 12.12.2020 17:00



History, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

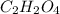
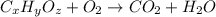
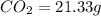
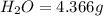
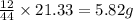 of carbon will be contained.
of carbon will be contained.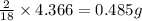 of hydrogen will be contained.
of hydrogen will be contained.