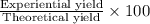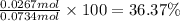Ammonia, nh3nh3 , can react with oxygen to form nitrogen gas and water. 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(l) 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(l) if 2.35 g2.35 g nh3nh3 reacts with 3.53 g3.53 g o2o2 and produces 0.650 l0.650 l n2n2 , at 295 k295 k and 1.01 bar1.01 bar , which reactant is limiting? o2(g)o2(g) nh3(aq)nh3(aq) calculate the percent yield of the reaction. percent yield:

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

You know the right answer?
Ammonia, nh3nh3 , can react with oxygen to form nitrogen gas and water. 4nh3(aq)+3o2(g)⟶2n2(g)+6h2o(...
Questions







History, 30.01.2020 18:45

Physics, 30.01.2020 18:45

Computers and Technology, 30.01.2020 18:45


English, 30.01.2020 18:45


Advanced Placement (AP), 30.01.2020 18:45

Mathematics, 30.01.2020 18:45


Mathematics, 30.01.2020 18:46

Mathematics, 30.01.2020 18:46

Social Studies, 30.01.2020 18:46

History, 30.01.2020 18:46

Mathematics, 30.01.2020 18:46

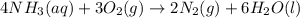
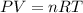
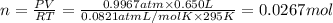
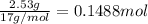
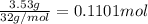
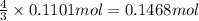 of ammonia.
of ammonia.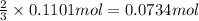 of nitrogen.
of nitrogen.