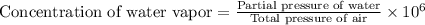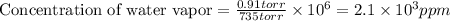Chemistry, 01.07.2019 23:30 itsyagirlbella
The concentration of water vapor in a sample of air that has a partial pressure of water of 0.91 torr and a total pressure of air of 735 torr is ppm. the concentration of water vapor in a sample of air that has a partial pressure of water of 0.91 torr and a total pressure of air of 735 torr is ppm. 0.81 0.12 8.1 ⋅ 10−4 1.2 1.2 ⋅ 103

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1


Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2
You know the right answer?
The concentration of water vapor in a sample of air that has a partial pressure of water of 0.91 tor...
Questions





Mathematics, 08.03.2021 05:50

History, 08.03.2021 05:50


Biology, 08.03.2021 05:50

Mathematics, 08.03.2021 05:50

Advanced Placement (AP), 08.03.2021 05:50

Biology, 08.03.2021 05:50

History, 08.03.2021 05:50


Mathematics, 08.03.2021 05:50

Mathematics, 08.03.2021 05:50

Mathematics, 08.03.2021 05:50


Chemistry, 08.03.2021 05:50


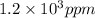
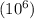 parts by the mass of the solution.
parts by the mass of the solution.