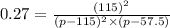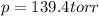Chemistry, 01.07.2019 23:30 NeverEndingCycle
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp = 0.27 at 700 k a reaction mixture initially contains equal partial pressures of no and cl2. at equilibrium, the partial pressure of nocl is 115 torr. what were the initial partial pressures of no and cl2 ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp...
Questions




Geography, 23.01.2020 17:31


Mathematics, 23.01.2020 17:31


Mathematics, 23.01.2020 17:31

English, 23.01.2020 17:31



Social Studies, 23.01.2020 17:31



Mathematics, 23.01.2020 17:31

History, 23.01.2020 17:31

Mathematics, 23.01.2020 17:31


Business, 23.01.2020 17:31

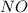 and
and 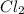 is 139.4 torr.
is 139.4 torr. 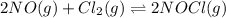
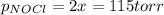
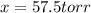
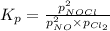
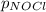 = 2x =115 torr
= 2x =115 torr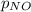 = (p-2x) = p-115 torr
= (p-2x) = p-115 torr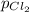 = (p-x)= p-57.5 torr
= (p-x)= p-57.5 torr