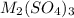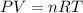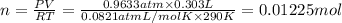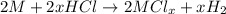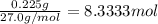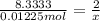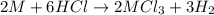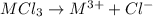Chemistry, 02.07.2019 00:10 naenae6775
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen (measured at 17°c and 741 mmhg) from an excess of hydrochloric acid. deduce from these data the corresponding equation and write formulas for the oxide and sulfate of m.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen...
Questions



Social Studies, 14.01.2020 00:31

Mathematics, 14.01.2020 00:31


History, 14.01.2020 00:31


Business, 14.01.2020 00:31






Mathematics, 14.01.2020 00:31


Biology, 14.01.2020 00:31




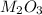 and sulfate of
and sulfate of