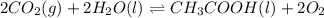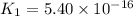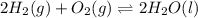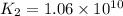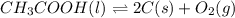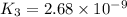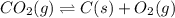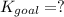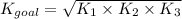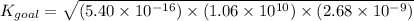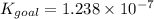Determine the value of the equilibrium constant, kgoal, for the reaction co2(g)⇌c(s)+o2(g), kgoal=? by making use of the following information: 1. 2co2(g)+2h2o(l)⇌ch3cooh(l)+2o2(g), k1 = 5.40×10−16 2. 2h2(g)+o2(g)⇌2h2o(l), k2 = 1.06×1010 3. ch3cooh(l)⇌2c(s)+2h2(g)+o2(g), k3 = 2.68×10−9

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
Determine the value of the equilibrium constant, kgoal, for the reaction co2(g)⇌c(s)+o2(g), kgoal=?...
Questions


Mathematics, 22.04.2020 03:42

English, 22.04.2020 03:42




Mathematics, 22.04.2020 03:42


Biology, 22.04.2020 03:42

Mathematics, 22.04.2020 03:42

Mathematics, 22.04.2020 03:42


Computers and Technology, 22.04.2020 03:42







Biology, 22.04.2020 03:43

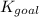 for the final reaction is,
for the final reaction is,