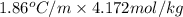Chemistry, 02.07.2019 01:20 lizzyhearts
What is the freezing point of water made by dissolving 22.78 g of ethylene glycol (ch2(oh)ch2(oh)) in 87.95 g of water? the freezing-point depression constant of water is 1.86 oc/m.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
What is the freezing point of water made by dissolving 22.78 g of ethylene glycol (ch2(oh)ch2(oh)) i...
Questions

Geography, 10.12.2020 06:10


Mathematics, 10.12.2020 06:10


Mathematics, 10.12.2020 06:10



Mathematics, 10.12.2020 06:10

Mathematics, 10.12.2020 06:10

History, 10.12.2020 06:10


History, 10.12.2020 06:10

Mathematics, 10.12.2020 06:10


Chemistry, 10.12.2020 06:10


Mathematics, 10.12.2020 06:20

Mathematics, 10.12.2020 06:20


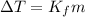
 = change in freezing point
= change in freezing point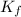 = freezing point depression constant
= freezing point depression constant 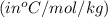
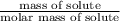
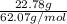
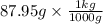 = 0.08795 kg
= 0.08795 kg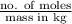
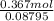
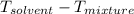 =
= 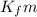
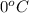 . Now, putting the given values as follows.
. Now, putting the given values as follows.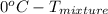 =
=