

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
Consider the two reactions. 2nh3(g)+3n2o(g)4nh3(g)+3o2(g)⟶4n2(g )+3h2o(l)⟶2n2(g)+6h2o(l) δ∘=−1010 kj...
Questions



Computers and Technology, 18.10.2019 13:30


English, 18.10.2019 13:30


Mathematics, 18.10.2019 13:30

Mathematics, 18.10.2019 13:30

Mathematics, 18.10.2019 13:30

History, 18.10.2019 13:30

Biology, 18.10.2019 13:30

Health, 18.10.2019 13:30

Mathematics, 18.10.2019 13:30

Biology, 18.10.2019 13:30

Social Studies, 18.10.2019 13:30





Computers and Technology, 18.10.2019 13:30

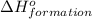 for the reaction is 591.9 kJ.
for the reaction is 591.9 kJ.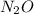 is:
is: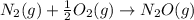
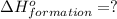
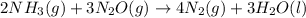
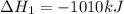 ( ÷ 3)
( ÷ 3)![4NH_3(g)+3O_2(g)\rightarrow 2N_2(g)+6H_2O(l) [tex]\Delta H_2=1531kJ](/tpl/images/0041/0429/f3925.png) ( ÷ 6)
( ÷ 6)![\Delta H^o_{formation}=[\frac{\Delta H_1}{3}]+[\frac{\Delta H_2}{6}]](/tpl/images/0041/0429/5c537.png)
![\Delta H^o_{formation}=[\frac{1010}{3}]+[\frac{1531}{6}]\\\\\Delta H^o_{formation}=591.9kJ](/tpl/images/0041/0429/fba80.png)


