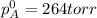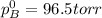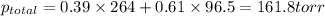Chemistry, 02.07.2019 04:10 NikkiZoeller
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at the same temperature. if 5.50 moles of liquid a and 8.50 moles of liquid b are combined to form an ideal solution, what is the total vapor pressure (in torr) above the solution at 20.0∘c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at...
Questions

Mathematics, 10.01.2020 06:31


















Social Studies, 10.01.2020 06:31

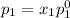 and
and 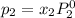
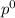 = pressure in the pure state
= pressure in the pure state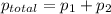
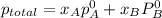
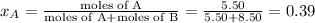 ,
, 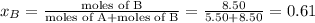 ,
,