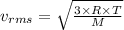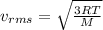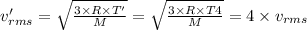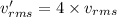If the absolute temperature of a gas is quadrupled, what happens to the root‑mean‑square speed of the molecules? nothing happens to the rms speed. the new rms speed is 16 times the original rms speed. the new rms speed is 4 times the original rms speed. the new rms speed is 2 times the original rms speed. the new rms speed is 1/4 the original rms speed.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
If the absolute temperature of a gas is quadrupled, what happens to the root‑mean‑square speed of th...
Questions


Mathematics, 30.07.2019 06:30

Health, 30.07.2019 06:30

Mathematics, 30.07.2019 06:30



Biology, 30.07.2019 06:30



Biology, 30.07.2019 06:30


Social Studies, 30.07.2019 06:30


Social Studies, 30.07.2019 06:30




Chemistry, 30.07.2019 06:30


Mathematics, 30.07.2019 06:30