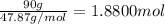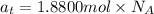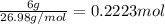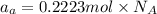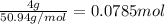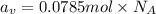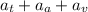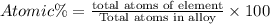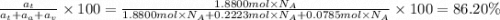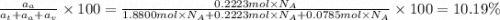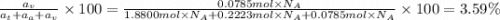Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1


You know the right answer?
The common titanium alloy known as t-64 has a composition of 90 weight% titanium 6 wt% aluminum and...
Questions

Mathematics, 09.12.2020 19:20


Mathematics, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20

History, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20


Social Studies, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20


History, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20

Health, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20

Mathematics, 09.12.2020 19:20