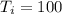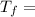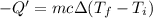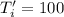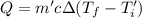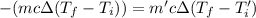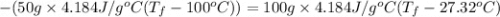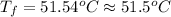Calculate the final temperature of the system: a 50.0 gram sample of water initially at 100 °c and a 100 gram sample initially at 27.32 °c are mixed. the specific heat of water is 4.184 j/gc). record your answer in scientific notation using 3 significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1
You know the right answer?
Calculate the final temperature of the system: a 50.0 gram sample of water initially at 100 °c and...
Questions

History, 14.12.2020 23:50





Spanish, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00

English, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00


English, 15.12.2020 01:00


Social Studies, 15.12.2020 01:00

Geography, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00