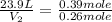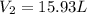Chemistry, 02.07.2019 19:20 azertyqwerty123
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l. the following reaction takes place: n2(g) + 2o2(g)2no2(g) calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l....
Questions

Mathematics, 19.02.2021 21:30



English, 19.02.2021 21:30

Social Studies, 19.02.2021 21:30


Mathematics, 19.02.2021 21:30


History, 19.02.2021 21:30

Mathematics, 19.02.2021 21:30

Mathematics, 19.02.2021 21:30



Mathematics, 19.02.2021 21:30



English, 19.02.2021 21:30

Mathematics, 19.02.2021 21:30

Business, 19.02.2021 21:30

Advanced Placement (AP), 19.02.2021 21:30

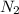 = 0.13 mole
= 0.13 mole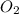 = 0.26 mole
= 0.26 mole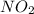 gas.
gas.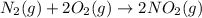
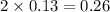 moles of
moles of 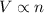
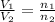
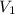 = initial volume of gas = 23.9 L
= initial volume of gas = 23.9 L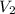 = final volume of gas = ?
= final volume of gas = ?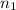 = initial moles of gas = 0.13 + 0.26 = 0.39 mole
= initial moles of gas = 0.13 + 0.26 = 0.39 mole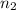 = final moles of gas = 0.26 mole
= final moles of gas = 0.26 mole