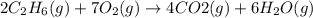What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g) recall that the coefficients of the final balanced equation should be whole numbers. thus, you might need to multiply through the equation by a factor of two to obtain whole numbers in your last step. if you have trouble balancing the equation below, use the first hint to view a video of a similar equation being balanced. then, use the rest of the hints to you balance the equation, step-by-step. express the coefficients as integers separated by commas. view available hint(s)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1


Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
What are the resulting coefficients when you balance the chemical equation for the combustion of eth...
Questions

Mathematics, 27.02.2021 03:00

Mathematics, 27.02.2021 03:00

History, 27.02.2021 03:00

Physics, 27.02.2021 03:00



Health, 27.02.2021 03:00


Mathematics, 27.02.2021 03:00

Mathematics, 27.02.2021 03:00


Mathematics, 27.02.2021 03:00



Mathematics, 27.02.2021 03:00

Mathematics, 27.02.2021 03:00




Mathematics, 27.02.2021 03:00