
Chemistry, 03.07.2019 20:30 dustonangiecook
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of an unknown hno3 solution required 29.1 ml of 0.200 m ba(oh)2 for complete neutralization. what is the concentration of the hno3 solution?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of...
Questions

Advanced Placement (AP), 19.05.2021 21:20


Mathematics, 19.05.2021 21:20


Mathematics, 19.05.2021 21:20






Engineering, 19.05.2021 21:20


Mathematics, 19.05.2021 21:20



History, 19.05.2021 21:20

Mathematics, 19.05.2021 21:20



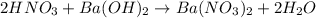
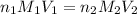
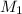 = molarity of
= molarity of 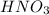 solution = ?
solution = ?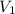 = volume of
= volume of 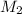 = molarity of
= molarity of 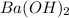 solution = 0.2 M
solution = 0.2 M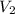 = volume of
= volume of 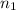 = valency of
= valency of 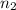 = valency of
= valency of 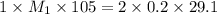
![M_1=0.11M[/tex] Therefore, the concentration of [tex]HNO_3](/tpl/images/0047/6355/af3c6.png) will be 0.11 M.
will be 0.11 M.

