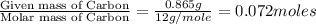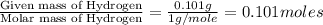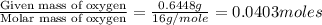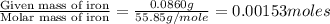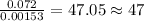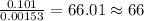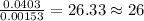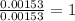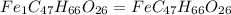Chemistry, 03.07.2019 21:20 delanieloya
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of co2 and 0.90829 g of h2o were produced. in a separate experiment to determine the mass percent of iron, 0.5446 g of the compound yielded 0.1230 g of fe2o3. what is the empirical formula of the compound?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1

Chemistry, 23.06.2019 14:10
What is true according to the second law of thermodynamics
Answers: 1
You know the right answer?
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of c...
Questions



English, 07.09.2021 19:00

Geography, 07.09.2021 19:00

Mathematics, 07.09.2021 19:00


Social Studies, 07.09.2021 19:00

Mathematics, 07.09.2021 19:00


Mathematics, 07.09.2021 19:00

History, 07.09.2021 19:00



Mathematics, 07.09.2021 19:00



Mathematics, 07.09.2021 19:00


Arts, 07.09.2021 19:00

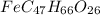
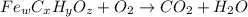
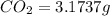
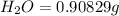
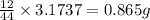 of carbon will be contained.
of carbon will be contained.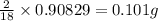 of hydrogen will be contained.
of hydrogen will be contained.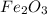 =
= 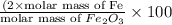
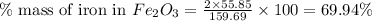
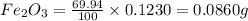 of iron.
of iron.