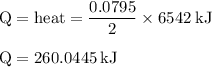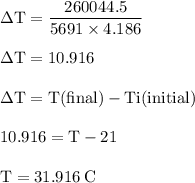Chemistry, 03.07.2019 21:20 tannerweberp5r8sg
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 6.200 g c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 6.200 g c...
Questions

Mathematics, 07.10.2019 10:50


Mathematics, 07.10.2019 10:50



Mathematics, 07.10.2019 10:50

Mathematics, 07.10.2019 10:50

English, 07.10.2019 10:50

History, 07.10.2019 10:50



Biology, 07.10.2019 10:50

Biology, 07.10.2019 10:50

Mathematics, 07.10.2019 10:50

History, 07.10.2019 10:50


Mathematics, 07.10.2019 10:50


History, 07.10.2019 10:50