Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3


Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
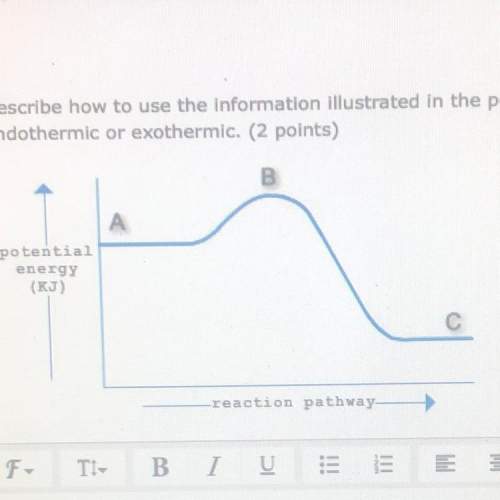
Describe how to use the information illustrated in the potential energy diagram below to determine t...
Questions


English, 25.01.2022 15:30


Mathematics, 25.01.2022 15:30

Mathematics, 25.01.2022 15:30

Mathematics, 25.01.2022 15:30



English, 25.01.2022 15:30

Mathematics, 25.01.2022 15:30

English, 25.01.2022 15:30



Mathematics, 25.01.2022 15:30

Mathematics, 25.01.2022 15:30

Computers and Technology, 25.01.2022 15:30

English, 25.01.2022 15:40


Mathematics, 25.01.2022 15:40

Social Studies, 25.01.2022 15:40