
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydrochloric acid to a sample of chalk and measuring the volume of carbon dioxide produced caco3(aq) + 2hcl -> cacl2(aq) + co2(g) + h2o(g) excess hydrochloric acid was added to 0.5g chalk and 100cm3 of carbon dioxide gas was given produced at r. t.p calculate the percentage purity of calcium carbonate in sample of chalk

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydr...
Questions

Mathematics, 12.08.2021 03:00

English, 12.08.2021 03:00

Mathematics, 12.08.2021 03:00

Mathematics, 12.08.2021 03:00




English, 12.08.2021 03:00

Mathematics, 12.08.2021 03:00




Law, 12.08.2021 03:00

Mathematics, 12.08.2021 03:00


History, 12.08.2021 03:00




Mathematics, 12.08.2021 03:00

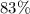 .
.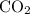 gas are released?
gas are released?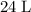 under room temperature and pressure (r.t.p,
under room temperature and pressure (r.t.p, 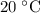 ,
, 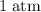 .) That's the same as
.) That's the same as 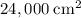 .
.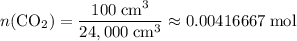 .
.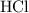 is in excess. How many moles of
is in excess. How many moles of 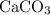 formula units will produce that
formula units will produce that 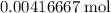 of
of 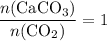 .
.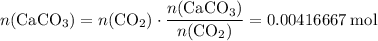 .
.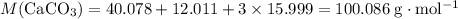 .
.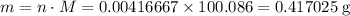 .
.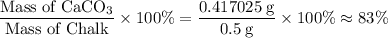 .
.

