Chemistry, 05.07.2019 05:20 claftonaustin846
Write the balanced chemical equation for the following acid and base reaction. (use the lowest possible whole number coefficients. include states-of-matter under the given conditions in your answer.)
hbr(aq) + lioh(aq) →
a) using the balanced reaction above, calculate the amount of 0.0024 m lioh that would neutralize 22 ml of 0.0026 m hbr.
b)how many moles of salt are produced in the reaction?
c)what is the molar concentration of the salt after the reaction is complete?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
Write the balanced chemical equation for the following acid and base reaction. (use the lowest possi...
Questions





Mathematics, 04.04.2020 11:31

Engineering, 04.04.2020 11:31


Mathematics, 04.04.2020 11:31

Chemistry, 04.04.2020 11:31

Health, 04.04.2020 11:31








Mathematics, 04.04.2020 11:32


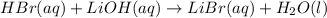
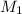 = 0.0024 M
= 0.0024 M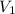
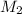 = 0.0026 M
= 0.0026 M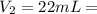
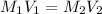
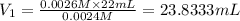
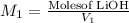
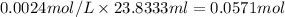
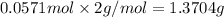
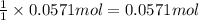 of LiBr
of LiBr