
Chemistry, 05.07.2019 05:20 Mw3spartan17
Which of the following values would you expect for the ratio of half-lives for a reaction with starting concentrations of 0.05m and 0.01m, t1/2(0.05m) / t1/2 (0.01m), if a reaction is known to be zero order?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

You know the right answer?
Which of the following values would you expect for the ratio of half-lives for a reaction with start...
Questions

Biology, 03.12.2020 04:20

Mathematics, 03.12.2020 04:20



Biology, 03.12.2020 04:20



Biology, 03.12.2020 04:20






Physics, 03.12.2020 04:20

French, 03.12.2020 04:20


Mathematics, 03.12.2020 04:20


Physics, 03.12.2020 04:20

![k=\frac{1}{t}([A_o]-[A])](/tpl/images/0052/8982/5bd85.png)
![[A_o]](/tpl/images/0052/8982/dc622.png) = initial concentration
= initial concentration![[A]=\frac{1}{2}[A_o]](/tpl/images/0052/8982/13a37.png)
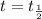 , the equation (1) becomes:
, the equation (1) becomes:![t_{\frac{1}{2}}=\frac{[A_o]}{2k}](/tpl/images/0052/8982/9ef84.png)
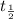
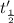
![\frac{t_{\frac{1}{2}}}{t_{\frac{1}{2}}'}=\frac{\frac{[0.05 M]}{2k}}{\frac{[0.01 M]}{2k}}=\frac{5}{1}](/tpl/images/0052/8982/a48c0.png)


