
Chemistry, 05.07.2019 06:10 cupkakekawaii45
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system? so2(g) + no2(g) ⇌ so3(g) + no(g) a) the reaction will shift in the direction of products. b) the reaction will shift to decrease the pressure. c) no change will occur since so3 is not included in the equilibrium expression. d) the reaction will shift in the direction of reactants. e) the equilibrium constant will decrease.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system?...
Questions

Mathematics, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01

History, 16.10.2020 19:01




English, 16.10.2020 19:01




History, 16.10.2020 19:01



Biology, 16.10.2020 19:01

Mathematics, 16.10.2020 19:01

Biology, 16.10.2020 19:01


Mathematics, 16.10.2020 19:01

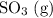 (analog to concentration in a solution.)
(analog to concentration in a solution.) 

