
Chemistry, 05.07.2019 14:10 ramirezzairap2u4lh
Areaction in which the rate depends linearly only on one reactant concentration is called

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2


Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Areaction in which the rate depends linearly only on one reactant concentration is called...
Questions



Physics, 23.06.2019 01:00




Biology, 23.06.2019 01:00

Physics, 23.06.2019 01:00


Mathematics, 23.06.2019 01:00


Mathematics, 23.06.2019 01:00

Mathematics, 23.06.2019 01:00




English, 23.06.2019 01:00


Mathematics, 23.06.2019 01:10

Chemistry, 23.06.2019 01:10

![\text{Rate} = k\cdot [\mathrm{A}]^{a}\cdot [\mathrm{B}]^{b}](/tpl/images/0054/3215/1d706.png) ,
, ![[\mathrm{A}]](/tpl/images/0054/3215/da066.png) and
and ![[\mathrm{B}]](/tpl/images/0054/3215/608bf.png) are the concentrations of the two reactants,
are the concentrations of the two reactants, 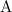 and
and 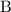 .
.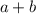 .
.![\text{Rate} = k\cdot [\mathrm{A}]](/tpl/images/0054/3215/c16bf.png) .
. " next to the concentration of
" next to the concentration of 

