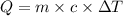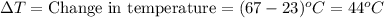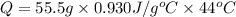Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
How much heat will be absorbed by a 55.5 g piece of aluminum (specific heat 0.930 j/g.°c) as it chan...
Questions

Mathematics, 11.12.2020 02:20

English, 11.12.2020 02:20




Mathematics, 11.12.2020 02:20


Mathematics, 11.12.2020 02:20



Mathematics, 11.12.2020 02:20

Mathematics, 11.12.2020 02:20


Arts, 11.12.2020 02:20




Mathematics, 11.12.2020 02:20

Mathematics, 11.12.2020 02:20

Mathematics, 11.12.2020 02:20