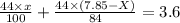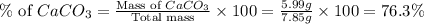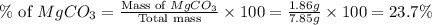Amixture contained calcium carbonate and magnesium carbonate in unspecified proportions. a 7.85g sample of this mixture has reacted with excess hydrochloric acid, producing 1.94l of carbon dioxide at 25 degrees c and 785mmhg. what are the percentage of calcium carbonate and magnesium carbonate in the sample?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1
You know the right answer?
Amixture contained calcium carbonate and magnesium carbonate in unspecified proportions. a 7.85g sam...
Questions

English, 08.01.2020 10:31

Mathematics, 08.01.2020 10:31

Chemistry, 08.01.2020 10:31

Spanish, 08.01.2020 10:31





Chemistry, 08.01.2020 10:31


Mathematics, 08.01.2020 10:31

History, 08.01.2020 10:31



Chemistry, 08.01.2020 10:31

Mathematics, 08.01.2020 10:31

Health, 08.01.2020 10:31

English, 08.01.2020 10:31


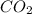 produced ca be calculated from ideal gas equation:
produced ca be calculated from ideal gas equation: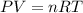
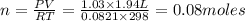
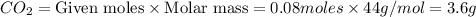
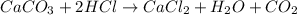
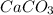 react to give 44 g of
react to give 44 g of 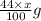 of
of 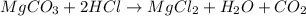
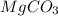 react to give 44 g of
react to give 44 g of 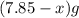 of
of 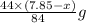 of
of