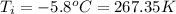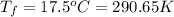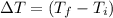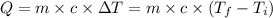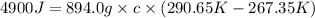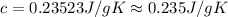Chemistry, 05.02.2020 13:44 salazarx062
Achemist carefully measures the amount of heat needed to raise the temperature of a 894.0g sample of a pure substance from −5.8°c to 17.5°c . the experiment shows that 4.90kj of heat are needed. what can the chemist report for the specific heat capacity of the substance? round your answer to 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Achemist carefully measures the amount of heat needed to raise the temperature of a 894.0g sample of...
Questions

Mathematics, 16.11.2020 22:00

English, 16.11.2020 22:00


Mathematics, 16.11.2020 22:00

Mathematics, 16.11.2020 22:00

English, 16.11.2020 22:00

French, 16.11.2020 22:00


Physics, 16.11.2020 22:00


Advanced Placement (AP), 16.11.2020 22:00


Biology, 16.11.2020 22:00

Biology, 16.11.2020 22:00



Mathematics, 16.11.2020 22:00


Mathematics, 16.11.2020 22:00