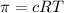Chemistry, 05.07.2019 18:20 janellesteele5918
5. tree sap can be a very concentrated solution of solutes in water. these are mostly sugars, with van’t hoff factors of 1. the root system provides a semi-permeable membrane across which water moves to “dilute” the sap, providing a significant osmotic pressure.
a. if a tree sap has an effective concentration of 37 molar, what pressure is generate at 298k across the endodermis root membrane? r = 0.08216 l. atm/mol. k.
b. if an osmotic pressure of 1.0 atm can raise a volume of water 10.33 meters high, how high can the sap of this tree rise?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
5. tree sap can be a very concentrated solution of solutes in water. these are mostly sugars, with v...
Questions



Chemistry, 28.09.2019 14:30



Mathematics, 28.09.2019 14:30

Physics, 28.09.2019 14:30

Advanced Placement (AP), 28.09.2019 14:30

Mathematics, 28.09.2019 14:30

Mathematics, 28.09.2019 14:30

Chemistry, 28.09.2019 14:30

Social Studies, 28.09.2019 14:30

Mathematics, 28.09.2019 14:30

Biology, 28.09.2019 14:30


History, 28.09.2019 14:30

Mathematics, 28.09.2019 14:30

Chemistry, 28.09.2019 14:30

Social Studies, 28.09.2019 14:30

History, 28.09.2019 14:30