Chemistry, 05.07.2019 18:20 brittnum9044
If the rate of a particular reaction is 4 ties faster at 373k that it was at 323k what is the activation energy for the reaction

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1


Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
If the rate of a particular reaction is 4 ties faster at 373k that it was at 323k what is the activa...
Questions


English, 13.01.2020 21:31


Mathematics, 13.01.2020 21:31

Mathematics, 13.01.2020 21:31

Health, 13.01.2020 21:31

English, 13.01.2020 21:31






Mathematics, 13.01.2020 21:31

English, 13.01.2020 21:31

English, 13.01.2020 21:31



English, 13.01.2020 21:31


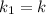
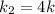
![\log \frac{k_2}{k_1}=\frac{E_a}{2.303\times R}[\frac{T_2-T_1}{T_2\times T_1}]](/tpl/images/0054/9720/494d5.png)
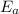 = Activation energy
= Activation energy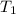 = Temperature when rate of the reaction was
= Temperature when rate of the reaction was 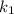
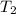 = Temperature when rate of the reaction was
= Temperature when rate of the reaction was 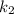
![\log \frac{4k}{k}=\frac{E_a}{2.303\times 8.314 J /mol K}[\frac{373 K-323K}{373 K\times 323 K}]](/tpl/images/0054/9720/0db3f.png)