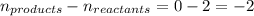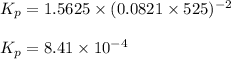Chemistry, 05.07.2019 18:20 aliceohern
Methanol can be synthesized from monoxide and hydrogen gas at 525 k. a reaction mixture consisting initially of 1.8 moles of co and 2.2 moles of h2 in 5.0-l container was found to contain 0.6 moles of ch3oh after reaching equilibrium (a) calculate equilibrium concentration (in molarity) of co and h2 (b) calculate equilibrium constants kc and kp for this reaction

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1


Chemistry, 23.06.2019 11:40
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2
You know the right answer?
Methanol can be synthesized from monoxide and hydrogen gas at 525 k. a reaction mixture consisting i...
Questions



Mathematics, 12.08.2020 05:01

Physics, 12.08.2020 05:01







Biology, 12.08.2020 05:01









 are 0.24 M and 0.32 M.
are 0.24 M and 0.32 M.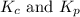 are 1.5625 and
are 1.5625 and 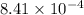
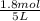
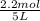
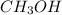 = 0.6
= 0.6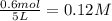
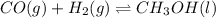
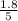
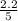 0
0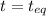
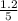
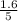
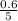
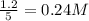
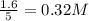
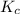 for the given chemical reaction follows:
for the given chemical reaction follows:![K_c=\frac{[CH_3OH]}{[CO][H_2]}](/tpl/images/0054/9747/63b2d.png)
![[CH_3OH]=0.12mol/L](/tpl/images/0054/9747/f965f.png)
![[CO]=0.24mol/L](/tpl/images/0054/9747/ee0b0.png)
![[H_2]=0.32mol/L](/tpl/images/0054/9747/aa037.png)
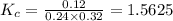
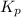 with
with 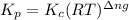
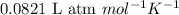
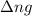 = change in number of moles of gas particles =
= change in number of moles of gas particles =