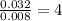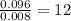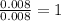Chemistry, 05.07.2019 18:20 hallmansean04
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after complete combustion, a 0.7020 g sample of the compound yields 1.4 g of co2, 0.86 g of h2o, and 0.478 g of sio2. what is the empirical formula of the compound?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after comp...
Questions





History, 22.07.2019 05:20



Mathematics, 22.07.2019 05:20

Mathematics, 22.07.2019 05:20




Chemistry, 22.07.2019 05:20




Chemistry, 22.07.2019 05:20


Computers and Technology, 22.07.2019 05:20

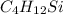 .
.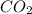 = 1.4 g
= 1.4 g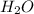 = 0.86 g
= 0.86 g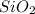 = 0.478 g
= 0.478 g 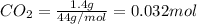
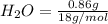 =0.048mol
=0.048mol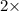 Moles of
Moles of 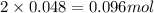
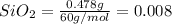 mol
mol