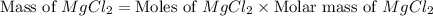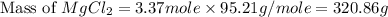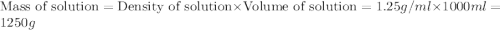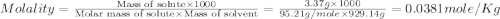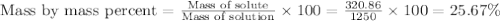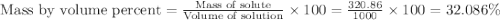Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
The density of a 3.37m mgcl2 (fw = 95.21) is 1.25 g/ml. calulate the molality, mass/mass percent, an...
Questions


History, 28.05.2020 05:58


Mathematics, 28.05.2020 05:58


Health, 28.05.2020 05:58

History, 28.05.2020 05:58

Mathematics, 28.05.2020 05:58

Mathematics, 28.05.2020 05:58


World Languages, 28.05.2020 05:58


History, 28.05.2020 05:58

Social Studies, 28.05.2020 05:58



Spanish, 28.05.2020 05:58


Mathematics, 28.05.2020 05:59

Mathematics, 28.05.2020 05:59

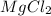 (solute) = 95.21 g/mole
(solute) = 95.21 g/mole