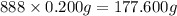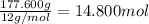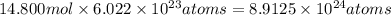The tiara worn by kate middleton for her wedding to prince william of england contains 888 diamonds and belongs to the british monarchy. if each diamond in the tiara is 1.0 carat, and given that diamond is a form of carbon and that 1 carat is defined as 0.200 g, calculate the number of atoms in the gemstones of that tiara.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
The tiara worn by kate middleton for her wedding to prince william of england contains 888 diamonds...
Questions

Mathematics, 09.01.2020 00:31





English, 09.01.2020 00:31






Physics, 09.01.2020 00:31





History, 09.01.2020 00:31



Geography, 09.01.2020 00:31

 .
.